Abstract
Background In polycythemia vera (PV), phlebotomy and low-dose aspirin are the standard of therapy in low-risk patients. However, whether phlebotomies are adequate to keep haematocrit (HCT) on target and if cytoreductive drugs should be added to reduce the still elevated risk of residual thrombosis, remains elusive. We present here the final results of the Low-PV phase II, investigator driven randomized trial (NCT030030025) testing the safety-efficacy profile of Ropeginterferon alfa-2b (Ropeg) versus phlebotomy-only program (standard therapy) in conventionally defined low-risk PV patients.
Methods Ropeg was administered subcutaneously every 2 weeks in a fixed dose of 100 micrograms, on top of standard therapy. The composite primary endpoint was the percentage of patients maintaining the median HCT values ≤45% over 12 months, in the absence of progressive disease (i.e., thrombosis, bleeding, progressive leukocytosis, symptomatic thrombocytosis, symptomatic splenomegaly or other uncontrolled symptoms). Secondary endpoints were number of phlebotomies, reduction of splenomegaly, leukocyte and platelet counts, iron deficiency, JAK2V617F allele burden, bone marrow-morphology and symptoms. Safety assessments were done at each monthly visit.
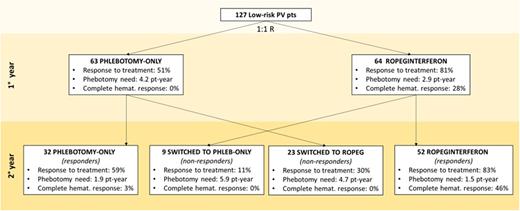
ResultsResponse to treatment A pre-planned interim analysis on 100 randomized patients followed-up for 1 year revealed that primary endpoint was already demonstrated and accrual of new patients was stopped by DSMB for overwhelming efficacy. The present analysis included 27 additional patients who, at the time of interim analysis, had not reached the first year of observation. Therefore, a total of 127 patients were followed up to 2 years (Ropeg arm, n=64 and standard arm, n=63), as per protocol. After the first year, the composite primary endpoint was achieved in 52 (81%) and 32 (51%) patients in the Ropeg and standard arm, respectively (p<0.001). Disease progression was seen in 8 patients (13%), all in standard arm, due to thrombocytosis progression with microcirculatory disturbances (n=6), cerebral TIA (n=1) and splenic vein thrombosis (n=1). The mean number of phlebotomies was 2.91 and 4.17 per pt/years in the Ropeg vs. standard arm, respectively (p=0.019); in 30% and 16% (p=0.064) zero or 1 phlebotomy was required; total phlebotomies were 186 vs. 263, respectively. Complete hematological response (CHR) was achieved in 28% in Ropeg arm and 0% in standard arm (Fig.). As per protocol, responding patients to Ropeg (n=52) or standard arm (n=32) continued the assigned treatment until month 24; complete response to treatment was found in 83% and 59%, respectively. This was associated with CHR in 46% and 3%, while the number of phlebotomies was the same in the two arms. Ropeg responders had a significant decrease of JAK2 VAF (-23%) particularly in those with initial VAF >50% (-56%). Non responders at 12 months were crossed-over to standard (n=9) or Ropeg (n=23). Only 30% of the latter met treatment response, none had CHR, the need of phlebotomies was high (4.7 per pt/year) and JAK2 VAF reduction was only 4%, compared to 12% obtained after 1 year of Ropeg administered since randomization. Only 1 patient who crossed-over to phlebotomy arm from Ropeg met a response. Results of centralized review of bone marrow biopsies at baseline and after 2 years will be presented at the meeting.
Adverse events and quality of lifeDuring 24 months, treatment related adverse events (AEs) were 48 (55%) and 36 (6%) in the Ropeg and standard arm, respectively, but no difference in the grading of AEs was seen. Ropeg discontinuation due to AEs was found in 14 patients (16%). Overall, moderate to severe symptoms were reported in 29% and 46% of Ropeg vs. standard arm, respectively (p=0.022).
Conclusion The Low-PV trial provided evidence that Ropeginterferon alfa-2b is superior to phlebotomy alone to keep low-risk patients with PV on HCT target and to limit disease progression. The safety profile was favorable and quality of life improved. While these results cannot prove a direct protective effect of Ropeg against thrombosis, they clearly show that it is effective on a set of potential surrogate endpoints that were correlated with thrombosis in the CYTO-PV study.
Disclosures
Barbui:AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Vannucchi:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphans Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; GSK: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Lectures. De Stefano:Bristol Myers Squibb/Celgene: Honoraria, Speakers Bureau; GlaxoSmithKline: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; AbbVie: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Carobbio:Novartis: Speakers Bureau. Rossi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ciceri:Kite Pharma: Consultancy. Iurlo:Novartis, BMS, Celgene, Incyte, Pfizer: Honoraria. Palandri:Sobi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AOP: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Sierra Oncology: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; CTI: Consultancy, Honoraria; Kartos/Telios: Consultancy, Honoraria. Pane:Novartis: Research Funding, Speakers Bureau. Siragusa:Csl Behring, Takeda, Amgen, Novartis, Bayer, Sobi, Novo Nordisk: Honoraria. Patriarca:Novartis: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Sanofi: Honoraria. Loscocco:Novartis: Speakers Bureau. Guglielmelli:Novartis, Abbvie: Other: Other member of advisory board, speaker at meeting. Rambaldi:Celgene-BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Omeros: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal